Throughout the blog, I have often talked about the fact I do scientific research as part of my PhD. However, I wanted to take this blog post as an opportunity to actually explain what my area of research is and how I do it. I got this idea when talking to people at my accommodation who I have probably bored with work stories (sorry about that). I realized they have no context to what I am saying and have not had the chance to see the equipment I get to use!
I will start by explaining that my research uses ultra-high vacuum (UHV) chambers and inside these is a surface that allows us to mimic an interstellar gain. You can see more about UHV chambers in this video so I will not explain them any further in this blog post.
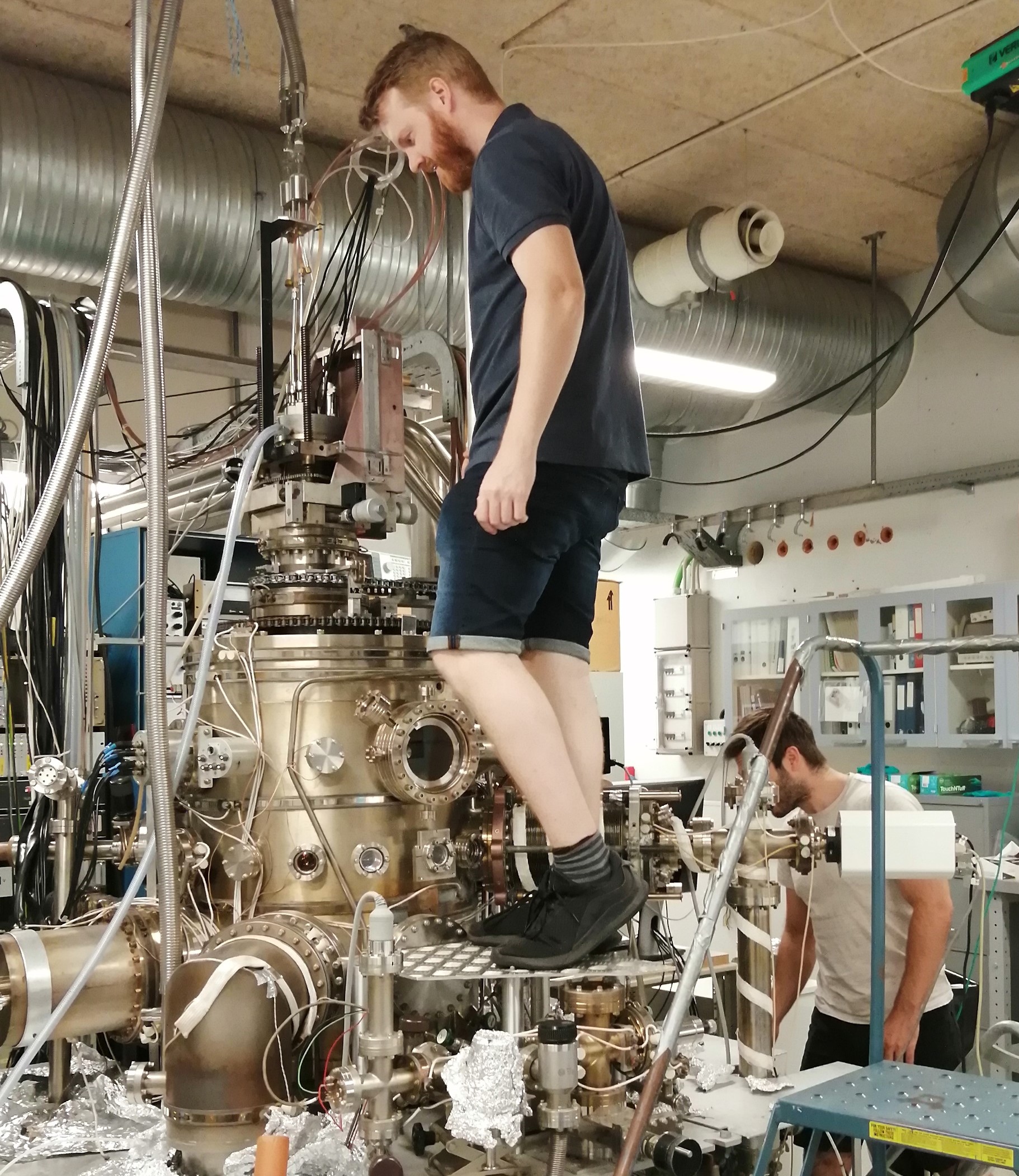
When I first arrived, I spent my time working with Frederik and Rijutha helping them with their research while learning how to use a UHV chamber and the different methods we can use to investigate reactions in interstellar space. At the beginning of this month, I stopped helping Frederik and have been able to start my own experiments. I aim to investigate putting glycine onto a surface and then exposing it to hydrogen to see what reactions occur. Believe me this sounds much easier than it actually is and I will explain why I am doing this, what glycine is and how I have been/plan to do this. I have been using the Big Chamber, which you can read more about here or just look at this photo of it.
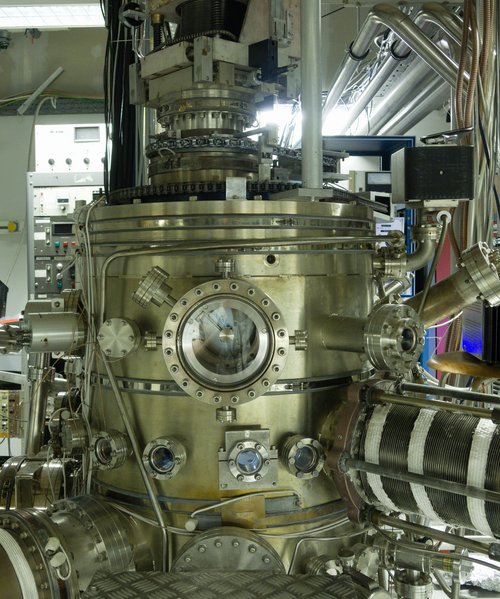
The Big Chamber
Glycine is an amino acid, which are known as building blocks of life. They are called this as they are the simplest component that joins together to form proteins that organisms need for life to function. Discovering if these molecules can form and then survive in the conditions found in interstellar space is necessary to answer the question of how life first originated. Hydrogen is found in these conditions and so seeing how it reacts with amino acids is a crucial component. I chose glycine as it is the simplest of the amino acids and so it is the easiest to form. You can see below what the structure of a single glycine molecule looks like.
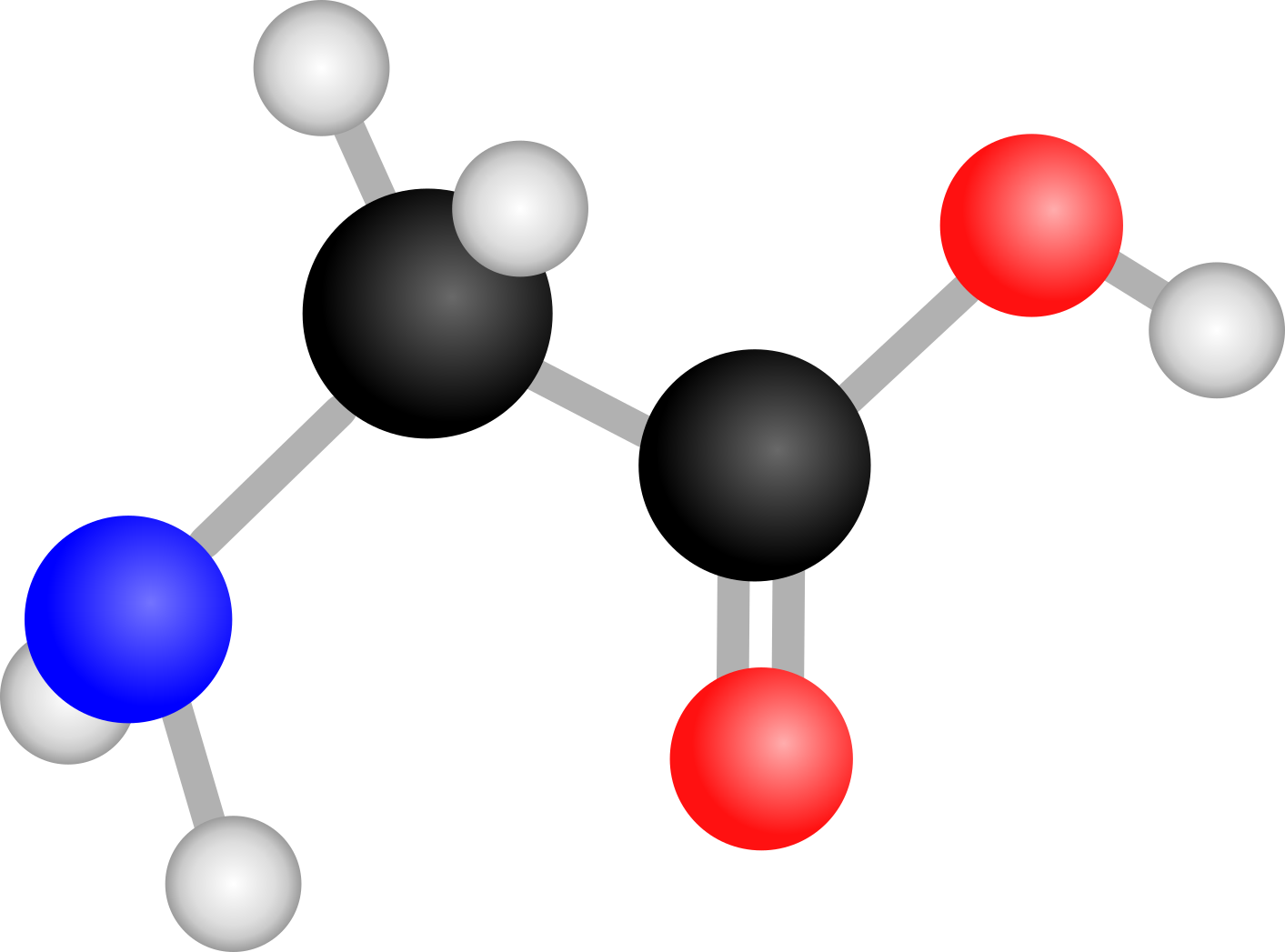
Glycine Molecule
I have put the glycine into a molecular doser which heats the glycine and causes it to be deposited onto the surface (step 1 in the figure below). It builds up on the surface to form a multilayer, which means it assembles into several layers. I then heat the surface causing all the layers apart from the first to be removed. This layer remains due to its stronger interactions with the surface. This single layer of glycine is called a monolayer and is the step I am currently at.
After I successfully do this I will expose the surface to the hydrogen for varying periods of time (step 2 in the figure below). Now I need a way of finding out what I have formed. To do this I am using a temperature programmed desorption which I have talked about before but is simply heating the surface such that the molecules desorb (are released from) the surface where I can then detect them using a mass spectrometer (step 3 in the figure below). I hope to see that the hydrogen has bonded to the glycine to form a radical and its mass is increased by one hydrogen mass.
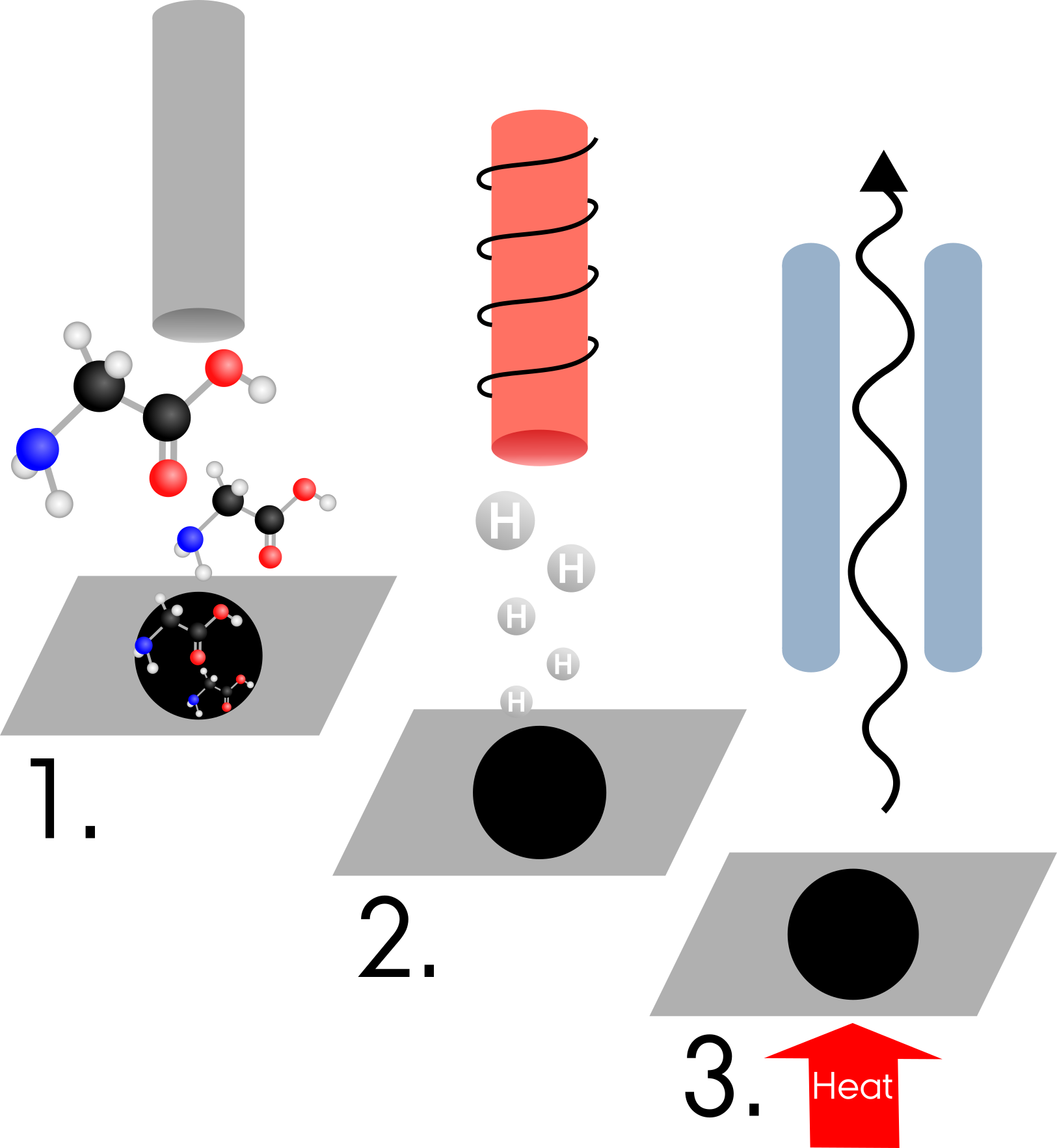
The process of a Temperature Programmed Desorption (TPD) measurement.
I will be doing more than just this for the next four years and I will use different techniques but it is a good feeling to have started on my own research! I now just need to do what I have said I will do in this blog post but I can assure you after being stuck in the UK for months being unable to do the experimental research I wanted it is very enjoyable to feel like I am progressing onto the real science work.