In the beginning of the month I have had the opportunity to visit the oldest city in the Netherlands, Nijmegen. Besides trying the exquisite local delicacy called marikenbrood, the supplementary purpose of the trip was to take part in experiments utilizing the Free-Electron Lasers for Infrared eXperiments (FELIX) laboratory. This facility is part of Radboud University and, as the name implies, provides free-electron lasers that can be employed to all sorts of research. In our case, we wanted to utilize it to explore photodesorption processes potentially taking place in interstellar space.

Entrance to the building where FELIX is located. Photo credit: https://www.wamenvanduren.nl/project/felix-hfml/

My fellow foodies out in the world, I implore you: try a piece of the decadent marikenbrood if you have the chance to. Photo credit: https://www.foodaholics.nl/lokale-zoethoudertjes-nijmeegs-marikenbrood/
According to chemical models, interstellar molecules should be largely frozen out onto icy grains during the early stages of star and planet formation. In fact, species such as CO and CO2 should be completely depleted in the gas phase at such cold and dense environments. The detection of gaseous CO in dark clouds and the midplanes of protoplanetary disks thus implies that some sort of desorption must take place, efficiently transporting these molecules from the surface to the gas.
Given the low temperatures associated with these clouds (~10 K), thermal desorption should be negligible. There is simply not enough thermal energy available to desorb the chemical species from the sticky icy surfaces. Therefore, non-thermal processes are paramount to explain astronomical observations of many molecules. Many different pathways for these processes have been suggested so far. Some of them are:
- Through photons impinging on the ice
- Through the impact of cosmic rays, which can result in desorption either by direct heating of the ice or by exciting and ionizing species along its path that subsequently release chemical energy
- As a result of exothermic reactions taking place in the ice
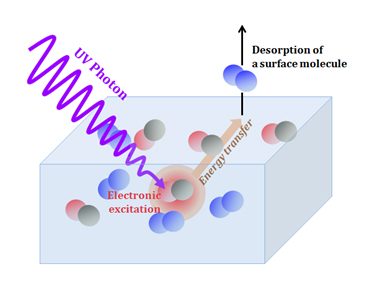
Scheme of UV-induced photodesorption. A CO molecule in the ice absorbs a UV photon and becomes excited. It transfers energy to the surface, resulting in the ejection of one N2 molecule from the ice. Figure credit: https://www.synchrotron-soleil.fr/en/news/why-do-telescopes-detect-gaz-where-there-should-only-be-dust-and-ice
Both theoretical and laboratory approaches have been extensively applied to study non-thermal desorption, which has been a constant topic of discussion due to its important implications on the chemistry of shielded interstellar regions. However, there are still many questions to be answered regarding the specific mechanisms through which these processes take place. So it goes without saying that I am very excited to be able to perform experiments so different than what I routinely do, and to explore this particularly hot—or, should I say, non-hot—astrochemical topic!
